Abstract
Introduction: De novo myelodysplastic syndromes (MDS) usually present in the elderly, within the context of the progressive acquisition of somatic mutations throughout the patient's life. On the other hand, MDS in children and younger adults are often associated with predisposing germline mutations. Early-onset MDS in adults (16-60 years old) accounts for »10% of all MDS cases. We aim to depict profiles of co-occurrence between germline and acquired variants in a Spanish multicenter cohort of patients with data from paired tumor-germinal whole exome sequencing (WES).
Methods: Prospective cohort study (2014-2021) of 197 patients from 25 Spanish Group of Myelodysplastic Syndrome (GESMD) centers with a de novo diagnosis of MDS and chronic myelomonocytic leukemia (CMML) between 16-60 yo, without prior organ dysfunction. In each case, we annotated 107 clinical variables in relation to family and personal history, diagnosis, treatment, HSCT and disease evolution. WES was performed in paired tumor-germline samples with 100x average depth, 150 million reads per sample, and >95% Phred Quality Score 30(Q30 a). WES libraries were prepared using SureSelectXT Target Enrichment System for Illumina Version B.2 and sequenced on a HiSeq4000-NovaSeq6000-Illumina platform. The analysis of the variants was carried out by means of an in-house pipeline. The germline variants were categorized according to American College of Medical Genetics and Genomics (ACMG) criteria.
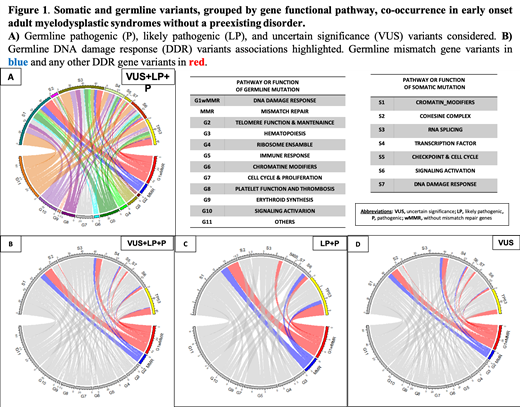
Results: The median age of the cohort at diagnosis was 49 years old (16-60) with the following WHO 2017 diagnoses: 13.7% MDS with single lineage dysplasia, 10.4% MDS with ring sideroblasts, 30.6% MDS with multilineage dysplasia, 17.9% MDS with excess blasts, 6% MDS with isolated del(5q), 2.2% MDS-unclassifiable, and 9.3% CMML. We found germline variants categorized as pathogenic (P), likely pathogenic (LP) or uncertain significance (VUS) in 83.2% (n=162) of 197 patients, with 24.4% (n=48) harboring LP and/or P variants.
A gene pathway-driven classification of germline variants (VUS+LP+P) categorized carrier patients as follows: 38.1% (n=75) with, at least, a variant in DNA damage response pathway (DDR), 2% (n=4) in telomere function maintenance (TF), 9.6% (n=19) in hematopoiesis regulators (HR), 6.1%(n=12) in ribosome function (RB), 14.7%(n=29) in immune response (IR), 13.3%(n=13) in chromatin modifiers, 14.2%(n=28) in cell cycle and proliferation (CP), 12.7%(n=25) in platelet function (PF), 7.1%(n=14) in erythroid synthesis (ES), and 22.8%(n=45) in signaling activation (SA) genes. Remarkably, out of 56 patients with germline variants in the DDR pathways other than MMR variants [DDRw/MMR GERM], 33 patients presented, at least, one somatic mutation. Twelve (36%) of these 33 cases involved an acquired variant in the TP53gene (TP53SOM). Within the DDR group, patients harboring germline variants in mismatch repair genes [MMR GERM](n=26) acquired, at least, one somatic mutation in 13 cases. In nine of these cases, the somatic variant was included included in the of chromatin modifying genes (ChrMod SOM) group.
Considering only pathogenic variants (LP+P): 8% (n=16) of patients showed, at least, a variant in DDR, 2% (n=1) in TF, 3% (n=6) in HR, 1.5% (n=3) in RB , 3.5%(n=7) in IR, 25% (n=5) in CP, 1% (n=2) in PF, 1%(n=2) in ES, and 3.5%(n=7) in SA genes. Within the DDR group, patients harboring MMR GERM variants (n=6) acquired, at least, one somatic mutation in 3 cases, of which 2 (67%) affected genes that were included in ChrMod SOMgenes,
Those patients with DDR GERM(VUS+LP+P)+TP53SOM variants showed lower levels of hemoglobin (9 g/dl vs. 11 g/dl, p=0.011), platelets (61x10 9/L vs 10 9x10 9/L, p=0.023) and a higher percentage of blasts in the BM (6% vs 3%, p=0.010). Moreover, they presented a worse outcome when applying a multivariate analysis with a dichotomized IPSS-R (≤3.5 vs >3.5): a shorter median time to AML (6 months vs. non-reached, p<0.001) and overall survival [OS](8.7 months vs. non-reached, p<0.001). Though a trend was noted, no statistical significance was reached when considering TP53SOM variants without co-occurring DDR GERM.
Conclusions: Young adult patients diagnosed with de novo MDS, without any previous organ dysfunction, show a germline profile enriched in variants affecting genes of the DDR pathways, frequently associated with TP53 acquired mutations, and showing significant clinical differences.
Tormo: Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Sanz: Boehringer Ingelheim: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Other: Travel, accommodations, and expenses; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, and expenses, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, and expenses; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Helsinn Healthcare: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, and expenses, Research Funding. Valcarcel: ASTELLAS: Consultancy, Honoraria, Speakers Bureau; TAKEDA: Consultancy, Honoraria, Speakers Bureau; AMGEN: Consultancy, Honoraria, Speakers Bureau; NOVARTIS: Consultancy, Honoraria, Speakers Bureau; JAZZ: Consultancy, Honoraria, Speakers Bureau; SANOFI: Consultancy, Honoraria, Speakers Bureau; SOBI: Consultancy, Honoraria, Speakers Bureau; BMS: Consultancy, Honoraria, Speakers Bureau; CELGENE: Consultancy, Honoraria, Speakers Bureau. Diez-Campelo: Takeda Oncology: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Jerez: GILEAD: Research Funding; BMS: Consultancy; Novartis: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal